Battery had become necessities of life since the invention of the voltaic battery more than 200 years ago. The dry battery, lead acid battery, solar battery and lithium ion battery are widely used in their specific applications nowadays, and lithium ion battery is the most favored one among them. Unlike nickel battery which have low capacity, memory effect, self-discharge and pollution problem, lithium ion battery has following merits:
- Environmentally.
- High power.
- High voltage.
- Excellent temperature resistance.
- Low self-discharge rate.
Structure and Working Principle of Lithium Ion Battery
The lithium ion battery can be divided into five main parts: cathode material, anode material, separator, electrolyte and case. As a kind of rechargeable battery, lithium ion battery work relies on lithium ion moving between cathode and anode. Lithium ion will repeat intercalate and de-intercalate between electrodes. During charging process, lithium ion will de-intercalate from cathode, and then intercalate anode after passed electrolyte, hence anode would be in lithium-rich state. For recharging process, the opposite occurs.
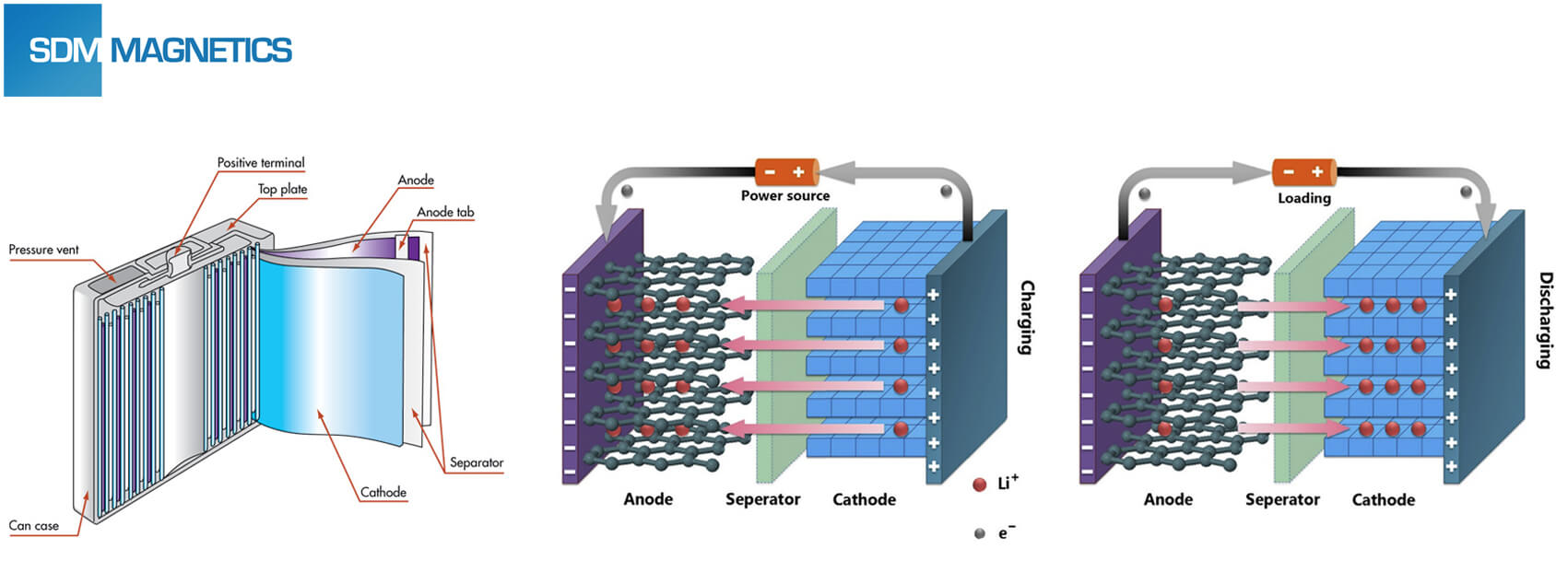
Classification of Lithium Ion Battery
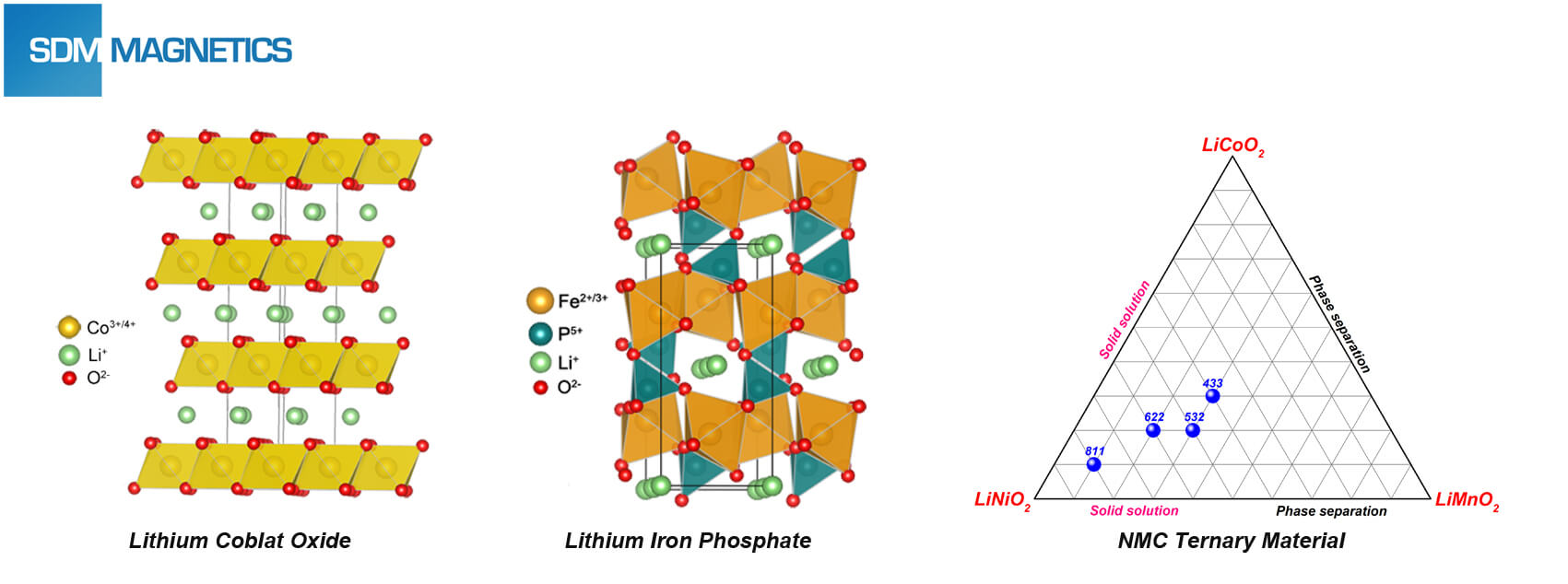
For lithium battery, the cathode material cost takes up 46% proportion of the total material cost. Arguably, performance and cost of lithium battery are directly decided by its cathode material. Normally, lithium ion battery can be classified by cathode material they use, includes:
- Lithium cobalt oxide battery: Excellent process performance, extremelyhigh compacting density, good volumetric energy density and charge-discharge property. High cost, insecurity (overcharge), bad circling life and material Lithium cobalt oxide batteries are widely served for 3C products (Computer, communication and consumer electronics).
- Lithium iron phosphate battery: Cheaper cost (rich raw material), excellent structure and temperature stability, outstanding security and circling life (quick charge). Bad process performance, undesirable volumetric and mass energy density. As a kind of power battery, Lithium iron phosphate batteries are widely applied in electric passenger vehicle and special vehicle.
- Lithium manganate battery: Bad high-temperature cycling performance can be enhanced by element doping. Lithium manganate battery can be used as power battery.
- NMC ternary battery: Ternary material combines advantages of LiNiO2, LiMnO2and LiCoO2, formed eutectic system of LiNiO2/LiMnO2/LiCoO2. NMC ternary batteries have been served to power battery of electric vehicle due to various merits, includes high energy density and cycling performance.
Impact of Lithium Ion Battery to Magnet Industry
The impact of lithium ion battery to magnet industry mainly from supply and price of cobalt. According to related statistic date, the battery industry constitutes a large part of cobalt consumption structure of the world (left) and China (right).
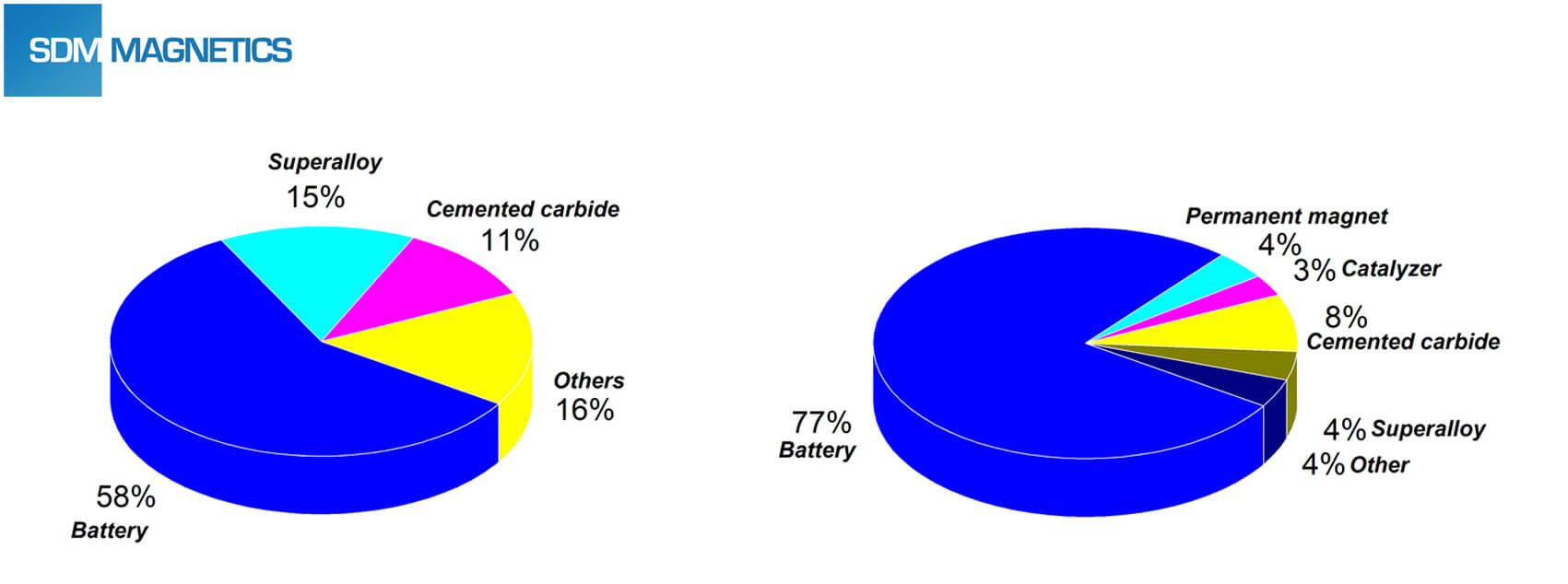
The customer base of 3C battery is very huge, and will be increased steadily for the indefinite future. 3C battery is still a decisive factor of world cobalt consumption. Power battery of the electric vehicle is also treated as a new growth point of cobalt consumption. It could be argued that the lithium battery industry has a significant impact on the supply and price of cobalt resource. Finally influenced the price of the permanent magnet with cobalt element, such as SmCo magnet and Alnico magnet.
After petroleum and rare earth, cobalt resource battle has been competed allover the world, and carmakers and consumer electronics firms have already layouts upstream supply chain of cobalt. Democratic Republic of Congo is the biggest cobalt reserves in the world.
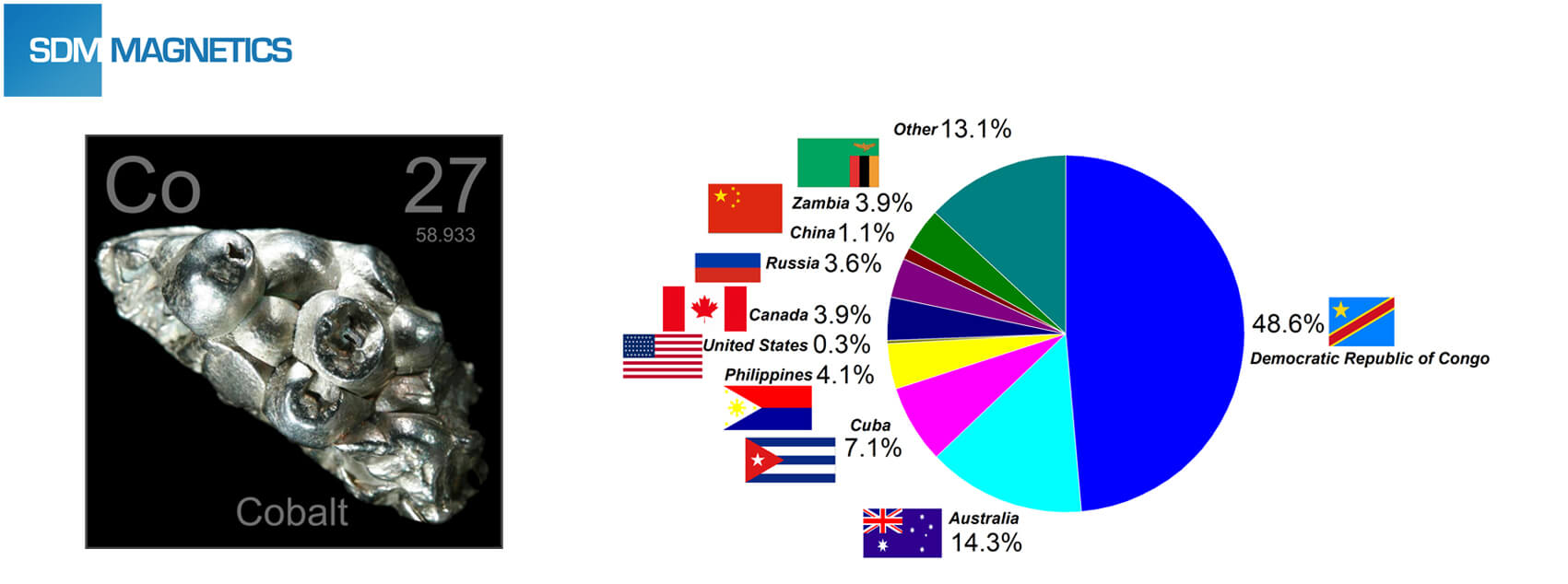
Meanwhile, the child labour of cobalt mines in Congo has also received widespread attention.

